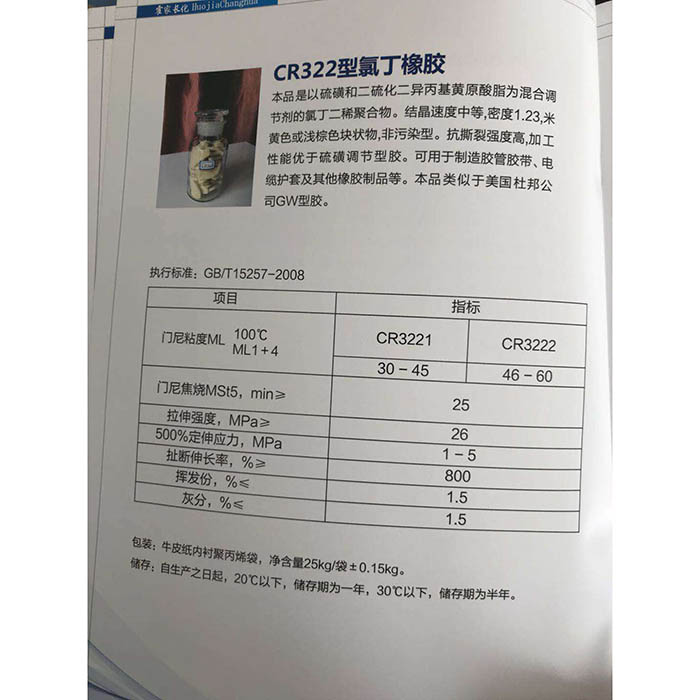
Sodium hydroxide
Sodium hydroxide, eo motsoako oa eona oa lik'hemik'hale e leng NaOH, hangata o tsejoa e le caustic soda, caustic soda le caustic soda. Ha e qhibiliha, e ntša monko oa ammonia. Ke caustic e matlaalkali, eo ka kakaretso e leng sebopeho sa flake kapa granular. E qhibiliha habonolo metsing (ha e qhibiliha ka metsing, e fana ka mocheso) 'me e etsa tharollo ea alkaline. Ho feta moo, e botsoa 'me e monya habonolo mouoane oa metsi (deliquescence) le carbon dioxide (ho senyeha) moeeng. NaOH ke e 'ngoe ea lik'hemik'hale tse hlokahalang lilaboratoring tsa lik'hemik'hale, hape ke e' ngoe ea lik'hemik'hale tse tloaelehileng. Sehlahisoa se hloekileng ha se na 'mala le kristale e bonaletsang. Boima ba 2.130 g/cm. Sebaka se qhibilihang ke 318.4 ℃. Sebaka se belang ke 1390 ℃. Lihlahisoa tsa indasteri li na le palo e nyane ea sodium chloride le sodium carbonate, e leng likristale tse tšoeu le tse opaque. Ho na le li-blocky, flaky, granular le sebopeho sa molamu. Mofuta oa bongata 40.01
Sodium hydroxidee ka sebelisoa e le moemeli oa ho hloekisa alkaline kalafo ea metsi, e qhibilihang ka ethanol le glycerol; Ha e kenelle ka har'a propanol le ether. E boetse e senya carbon le sodium mochesong o phahameng. Karabelo e sa lekanang le halogen joalo ka chlorine, bromine le iodine. Neutralize ka li-acids ho etsa letsoai le metsi.
Lintho tsa 'mele tsa ho phutha
Sodium hydroxide ke 'mala o mosoeu o khanyang oa kristale. Tharollo ea eona ea metsi e na le tatso ea astringent le maikutlo a sany.
Bolotsana bo menahaneng Bo na le bohlasoa moeeng.
Ho monyela ha metsi
Alkali e tiileng e na le hygroscopic haholo. Ha e pepesehile moeeng, e monya limolek'hule tsa metsi moeeng, 'me qetellong e qhibiliha ka ho feletseng ka tharollo, empa metsi a sodium hydroxide ha a na hygroscopicity.
Solubility e menang
Ho mena alkalinity
Sodium hydroxide e tla arohana ka ho feletseng ho li-ion tsa sodium le li-ion tsa hydroxide ha e qhibiliha ka metsing, kahoo e na le kakaretso ea alkali.
E ka etsa karabelo ea acid-base neutralization le protonic acid efe kapa efe (eo hape e leng karolo ea karabelo ea ho bola habeli):
NaOH + HCl = NaCl + H₂O
2NaOH + H₂SO₄=Na₂SO₄+2H₂O
NaOH + HNO₃=NaNO₃+H₂O
Ka mokhoa o ts'oanang, tharollo ea eona e ka ba le karabelo ea ho bola habeli ka tharollo ea letsoai:
NaOH + NH₄Cl = NaCl +NH₃·H₂O
2NaOH + CuSO₄= Cu(OH)₂↓+ Na₂SO₄
2NaOH+MgCl₂= 2NaCl+Mg(OH)₂↓
Ho mena ha saponification
Liphetohong tse ngata tsa manyolo, sodium hydroxide le eona e bapala karolo e ts'oanang le ts'usumetso, eo har'a eona e emelang haholo ke saponification:
RCOOR' + NaOH = RCOONA + R'OH
Hlakola tse ling
Lebaka leo ka lona sodium hydroxide e senyehang habonolo hore e be sodium carbonate (Na₂CO₃) moeeng ke hobane moea o na le carbon dioxide (co):
2NaOH + CO₂ = Na₂CO₃ + H₂O
Haeba carbon dioxide e ntse e tsoela pele ho hlahisoa, sodium bicarbonate (NaHCO₃), e tsejoang ka hore ke baking soda, e tla hlahisoa, 'me equation ea karabelo e tjena:
Na₂CO₃ + CO₂ + H₂O = 2NaHCO₃
Ka ho tšoanang, sodium hydroxide e ka itšoara ka li-acidic oxides tse kang silicon dioxide (SiO₂) le sulfur dioxide (SO):
2NaOH + SiO₂ = Na₂SiO₃ + H₂O
2 NaOH+SO (trace) = Na₂SO₃+H₂O
NaOH+SO₂ (e feteletseng) = NaHSO₃ (e hlahisitsoe NASO le metsi a itšoara ka SO e feteletseng ho hlahisa nahSO)